In order to maintain a healthy HbA1C, type 1 diabetics need to be
able to quickly,
accurately and consistently determine and track their glucose levels and predict imminent and overall
Δglucose. This can help the patient prevent hypo- and hyperglycemic events, the former of which occurs
approximately twice a week. (Lin et al., 2023) Existing market Continuous Glucose Monitoring devices
(CGMs) are able to achieve this (ADCES Staff Writer, 2024) but the medical adhesives used by them can
cause skin irritation. (Messer et al., 2018) Current generation CGMs also require peripheral devices,
such as a mobile device, and an NFC connection to display, store and share data with family members and
healthcare practitioners.
Type 1 diabetics are able to check their glucose levels through
two methods,either blood glucose,
measured manually, or interstitial fluid glucose, measured via CGM. Manual blood testing can cause
scarring on the fingers, which makes it progressively harder to test blood glucose, which leads to test
infrequency. Test infrequency leads to poorly managed glucose levels and more frequent hyper- and
hypoglycemic events, as opposed to the average of two-four events per week. Manual blood tests also
require a blood testing kit, consisting of a short spring loaded needle, an absorbent strip and blood
glucose analysis device, which means that diabetic patients need to carry these peripherals with them at
all times, which can get in the way of certain lifestyles and activities.
Type 1 diabetics are able to check their glucose levels through two methods,either blood glucose,
measured manually, or interstitial fluid glucose, measured via CGM. Manual blood testing can cause
scarring on the fingers, which makes it progressively harder to test blood glucose, which leads to test
infrequency. Test infrequency leads to poorly managed glucose levels and more frequent hyper- and
hypoglycemic events, as opposed to the average of two-four events per week. Manual blood tests also
require a blood testing kit, consisting of a short spring loaded needle, an absorbent strip and blood
glucose analysis device, which means that diabetic patients need to carry these peripherals with them at
all times, which can get in the way of certain lifestyles and activities.
This causes problems when patients need frequent data to maintain a healthy HbA1C, and a poor HbA1C can
lead to keto-acidosis, necrotic tissue in the extremities, and potentially, death.
Ideally, patients would not need any assistance to maintain an HbA1C of 6.5%
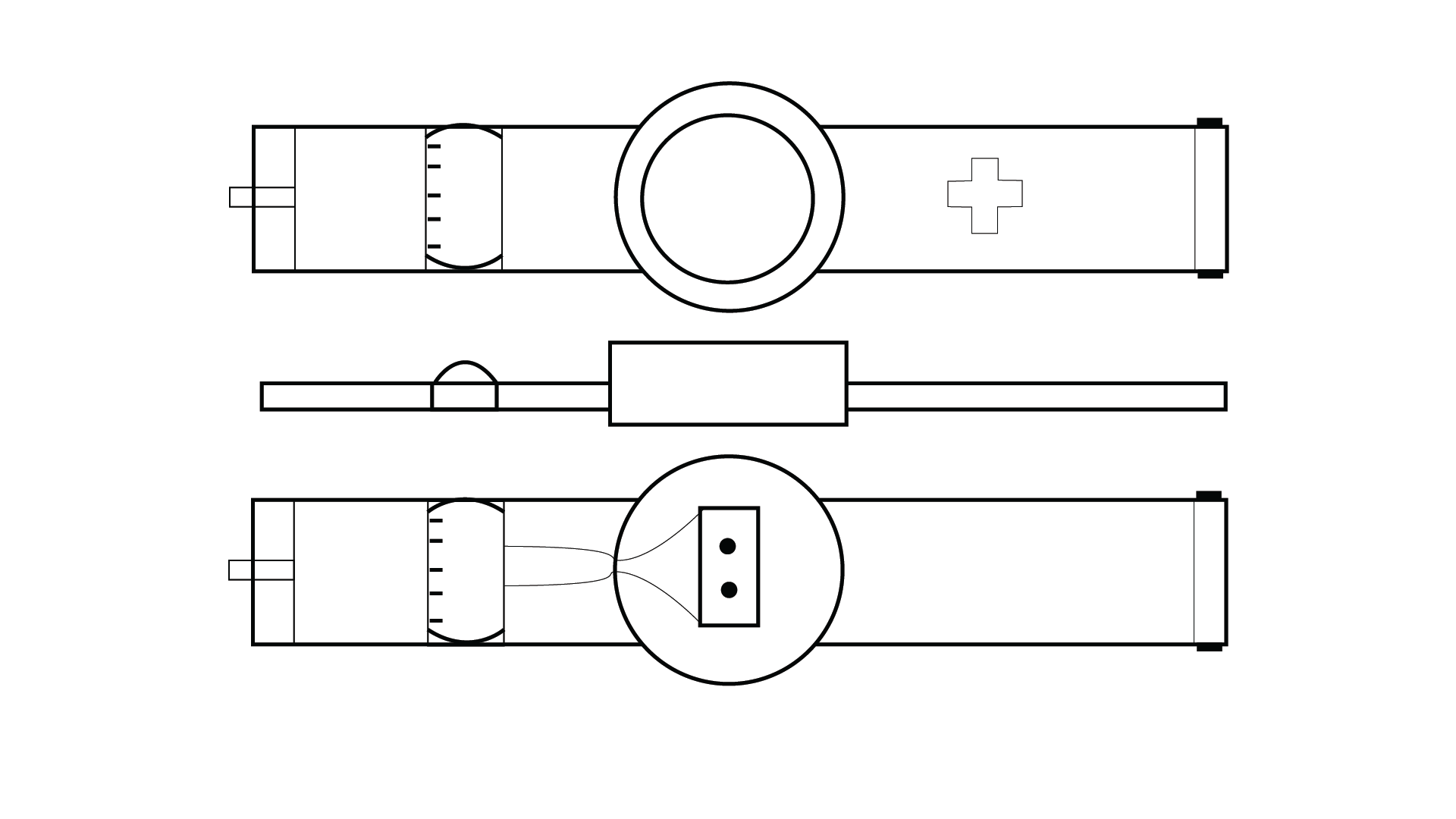
The Objectives of the project are to create a device that can act as an
implantable CGM device, that
does not need to use adhesives, and that can be continuously used without replacement. Secondary
objectives for the device would be to have hypoglycemic response Glucose injection, and an added CSII
device.
The project aims to lessen the prevalence of poor-HbA1C health complications, as well as comfortably
lower the average HbA1C to the 6-7% range, which is considered the diabetic ideal range.
The project will attempt to achieve this aim by method of the following objectives: Increase the usage
of CGM systems within the diabetic population, Increase the sampling value of the average glucose
trajectory tests in order to present clearer and more accurate results, and respond automatically to
hypoglycemic and hyperglycemic events, to increase time-in-range. To achieve these objectives, the
product needs to have an inegrated CSII and glucose injection system, be attached to the epidermis in a
way that does not irritate the skin, be easily readable to the diabetic patient, and be entirely
self-contained.
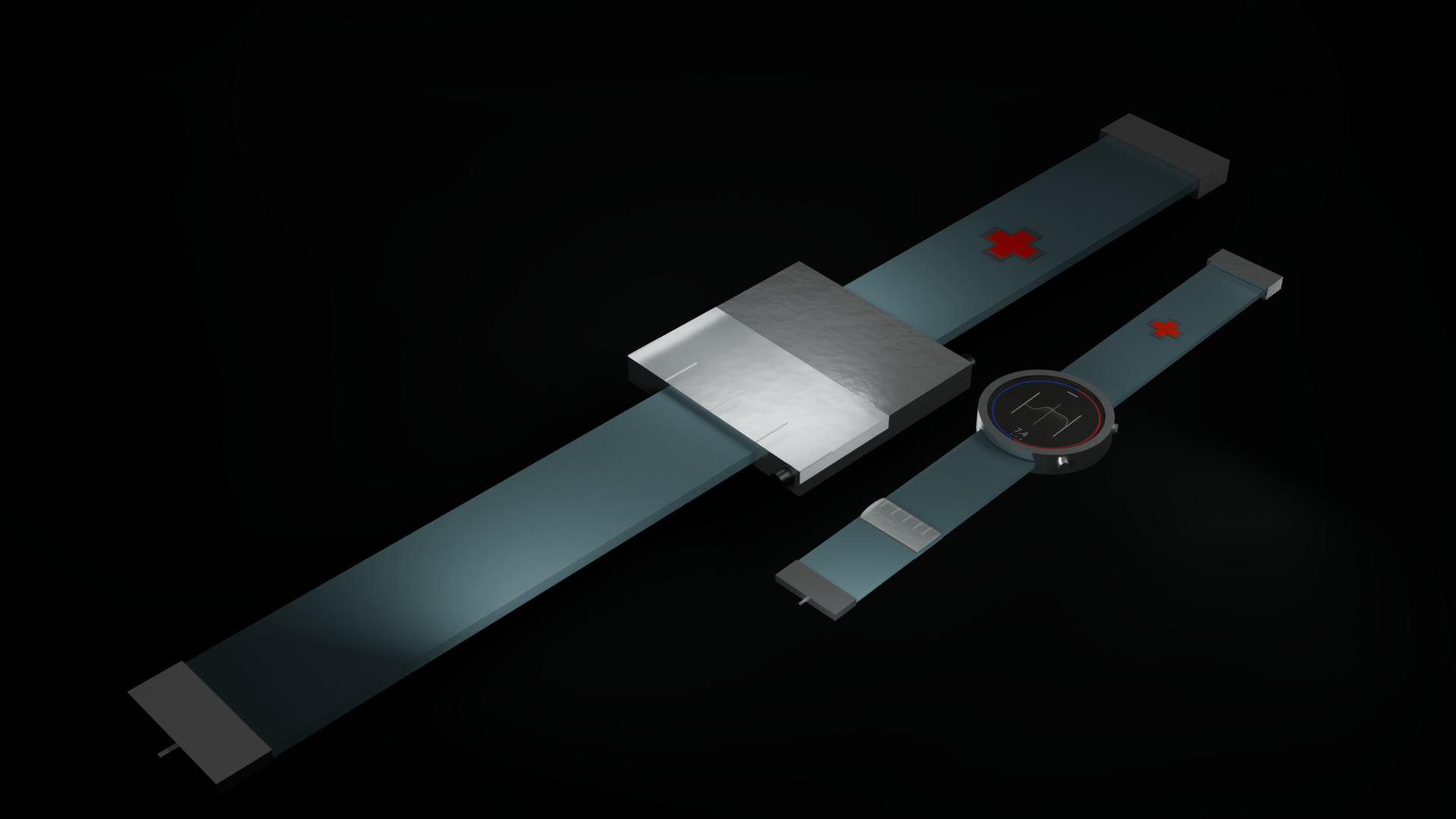
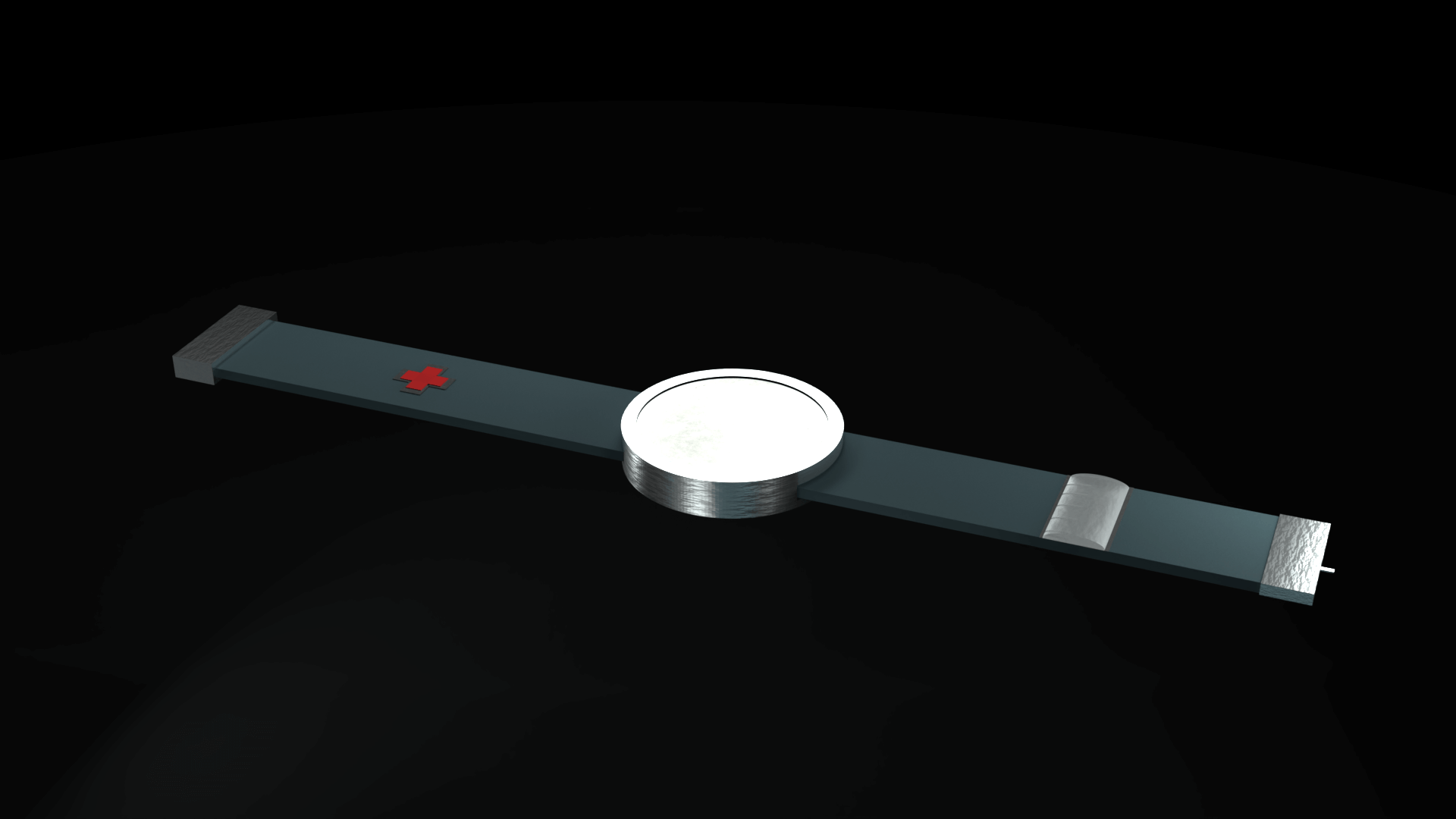
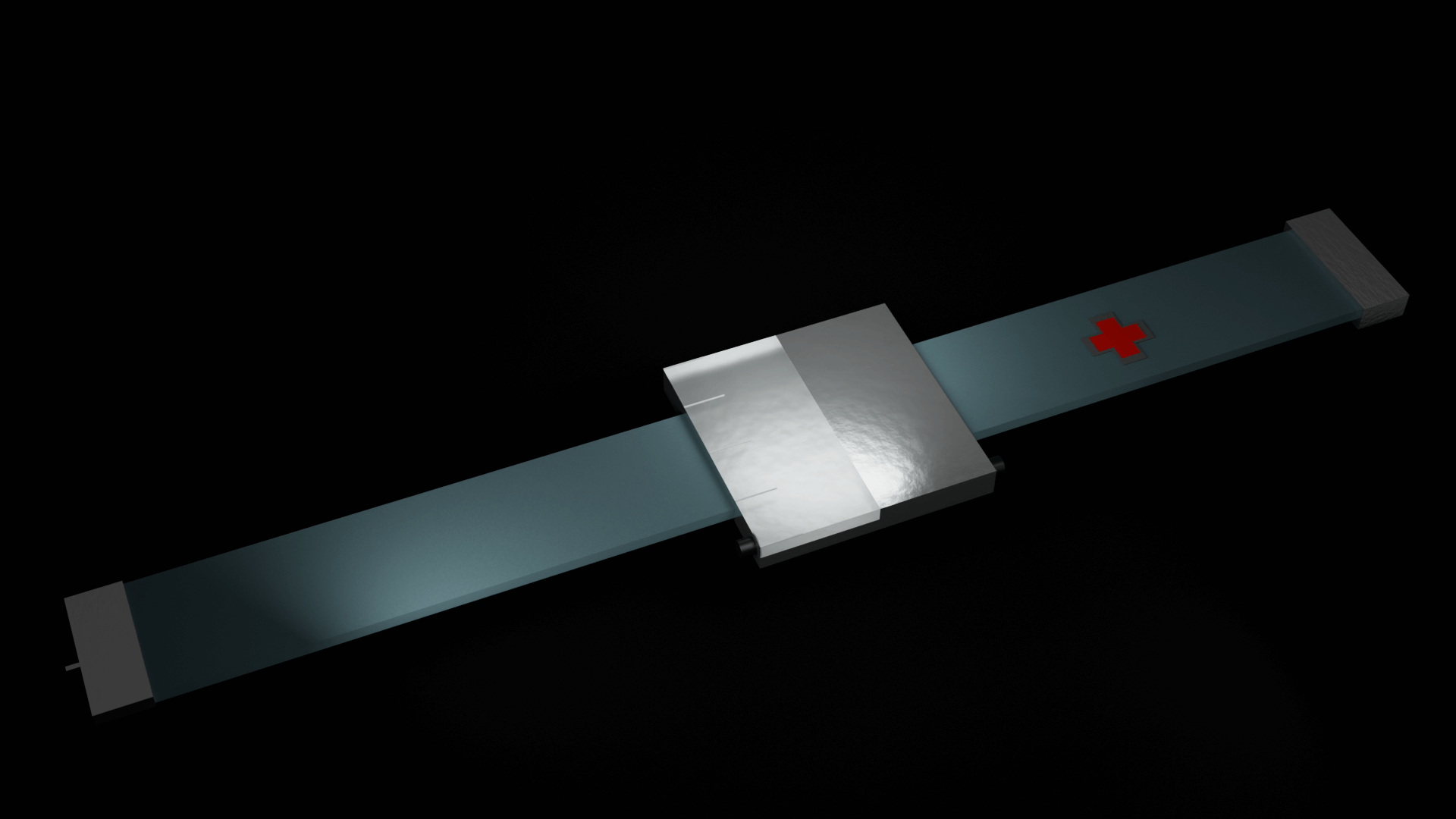
In order to fulfill the requirements as stated above, the CGM/CSII system would
have to be a wearable technology. This would have to be developed to be functional and stylish, as well
as
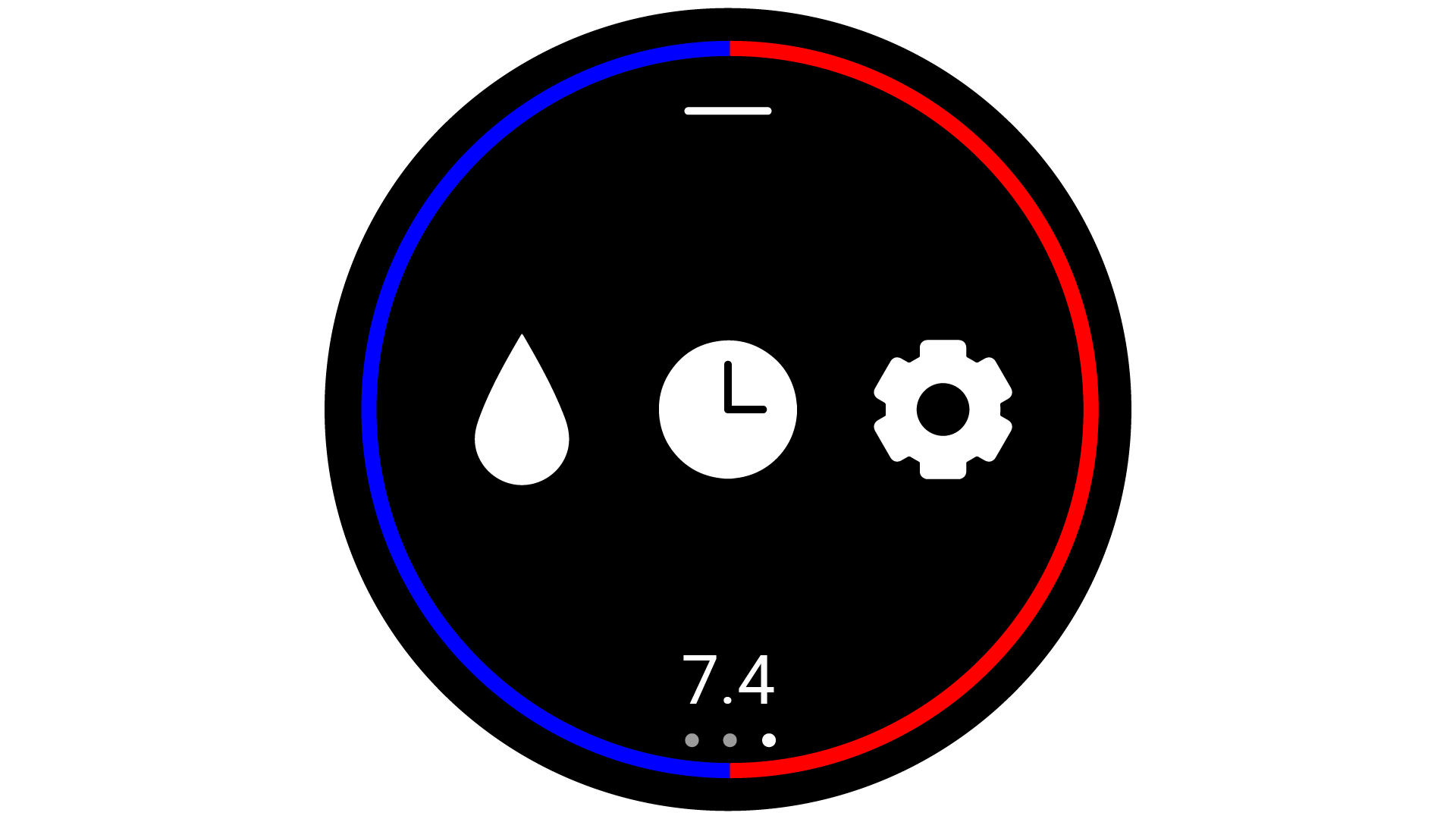
have a simple to use User Interface. The product was then designed as a watch/ armband combo, which
could be
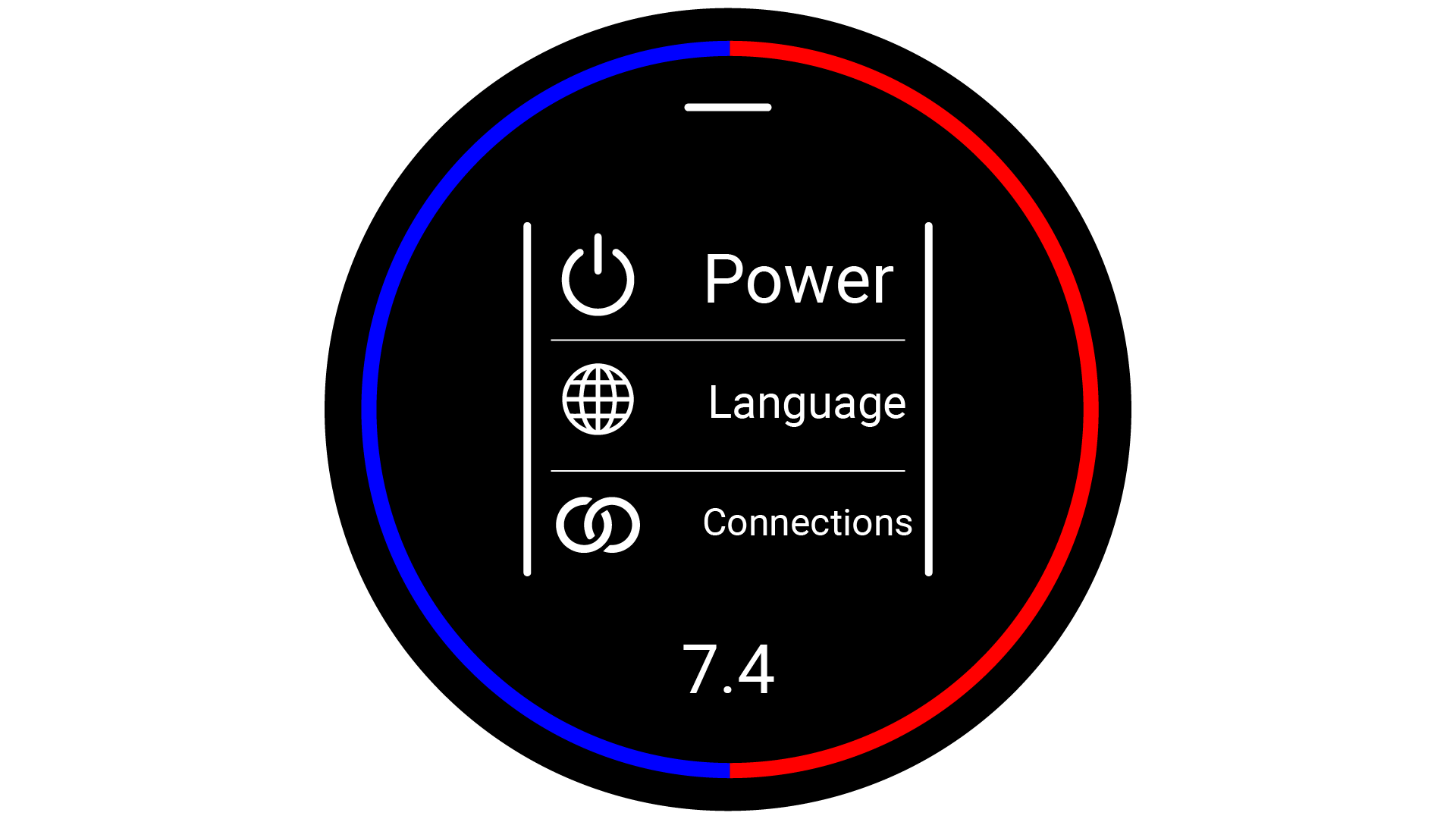
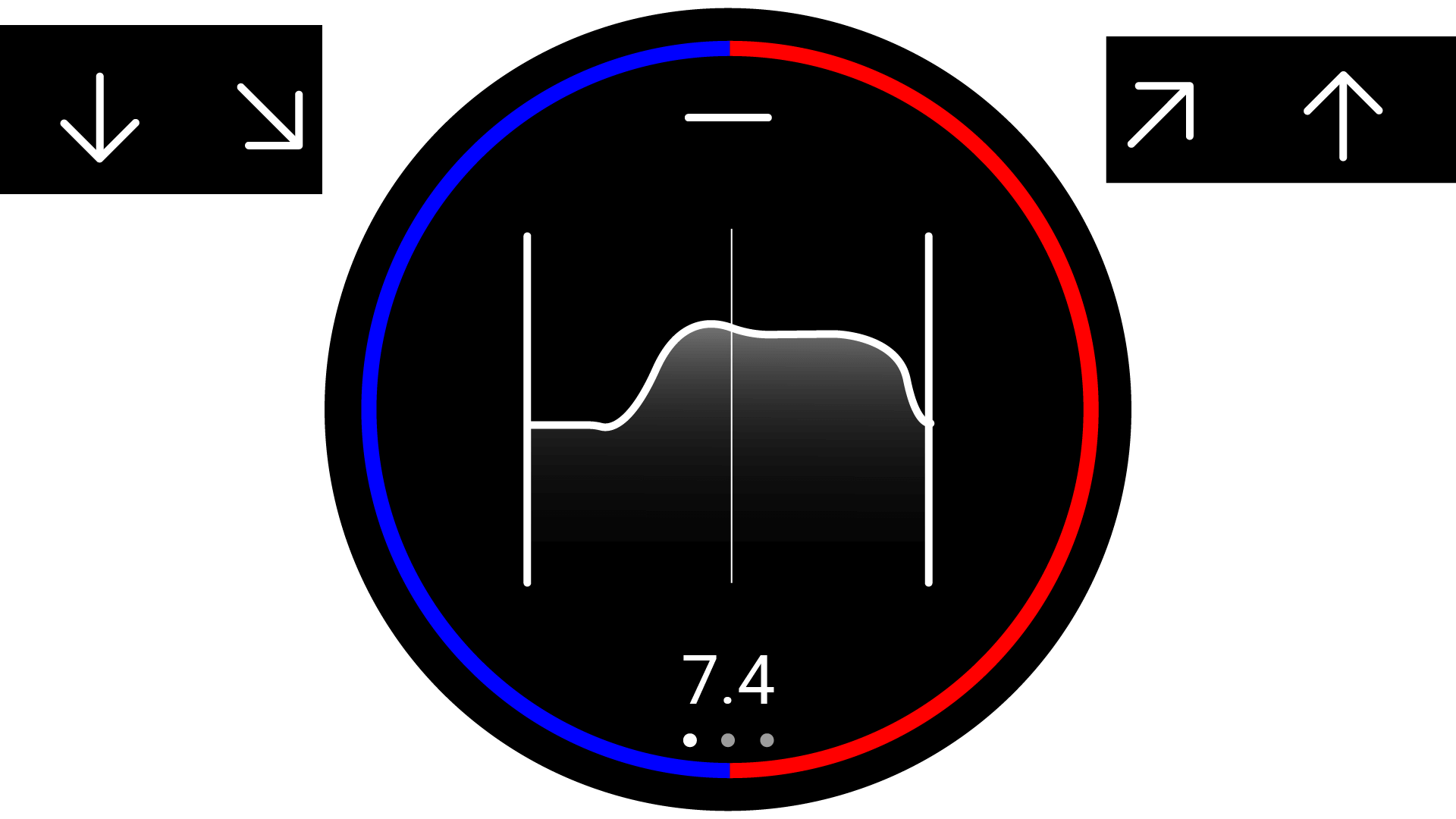
interacted with by interfacing with the watch-face. The interface was then designed to fit a circular
screen, and be interacted with via touch screen gestures. The user interface was designed to use as
little
text as possible, favouring simple icons, so that the user would not struggle to read the interface
quickly.